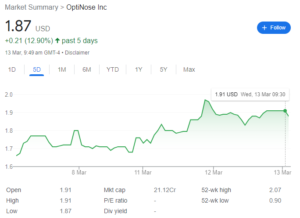
Optinose Inc. (OPTN) is a specialty pharmaceutical company focused on creating and bringing to market innovative products for patients with diseases treated by ear, nose, and throat (ENT) and allergy specialists. It’s mission is to improve the quality of life for patients.
Financials:
- Revenue: XHANCE net revenue for Q4 2023 was $19.9 million, down 5% YoY.
- Expenses: Research & Development (R&D) and Selling, General & Administrative (SG&A) expenses combined decreased 31% YoY to $85.1 million in 2023.
- Net Loss: The company reported a net loss of $35.5 million for 2023, or $0.32 per share.
- Cash Position: Optinose has $73.7 million in cash and cash equivalents as of December 31, 2023.
Recent News:
- Optinose reported Q4 and full-year 2023 financials.
- XHANCE (nasal corticosteroid) net revenue for 2023 was $71.0 million, a decrease of 7% YoY.
- The company awaits a PDUFA target action date of March 16, 2024, for its sNDA seeking approval of XHANCE for chronic sinusitis.
- Chronic sinusitis is 10 times more prevalent than XHANCE’s current nasal polyps indication.
- Other recent news
Positive Developments:
- PDUFA target action date approaching: Optinose’s sNDA for XHANCE to treat chronic sinusitis has a PDUFA target action date of March 16, 2024. Approval would significantly expand the addressable market for XHANCE. Chronic sinusitis is diagnosed 10 times more frequently than XHANCE’s current nasal polyps indication, and there is currently no FDA-approved medication for it.
- Successful cost management: Optinose reduced SG&A and R&D expenses by 31% in 2023 compared to 2022, demonstrating improved operational efficiency.
- Cash runway: The company has $73.7 million in cash and cash equivalents, which can help support operations and potential launch activities.
Strengths:
- Unique product: XHANCE has the potential to be the first FDA-approved medication for a much larger chronic sinusitis market.
- Experienced team: Management has a strong background in the ENT and allergy space.
- Improved financials: Reduced operating expenses demonstrate improving efficiency.
Weaknesses:
- Limited revenue: Reliant on XHANCE sales with a small market penetration currently.
- Negative cash flow: The company is not yet profitable and requires external funding.
- Dependence on regulatory approval: Future success hinges on FDA approval for the broader chronic sinusitis indication.
Opportunities:
- Large addressable market: Chronic sinusitis affects millions of patients, offering significant growth potential.
- Potential for expanded product line: Optinose’s technology could be applied to other ENT indications.
- Strategic partnerships: Collaboration with larger pharmaceutical companies could boost sales and marketing reach.
Threats:
- Competition: Existing treatments and potential new entrants could hinder market share.
- Regulatory hurdles: Delays or rejections in the ongoing sNDA approval process would be a major setback.
- Reimbursement challenges: Securing adequate insurance coverage for XHANCE is crucial for patient access.
Future Outlook:
Optinose’s future hinges on the upcoming PDUFA decision for the chronic sinusitis indication. Approval would unlock a significantly larger market opportunity and potentially lead to increased revenue and profitability.
Notes:
- The company expects XHANCE net revenue for Q1 2024 to be around $13.0 million.
- The average net revenue per prescription for 2024 is projected at $220.
Disclaimer: This analysis is for informational purposes only and should not be considered financial advice. Please consult with a financial advisor before making any investment decisions.
Additional Resources:
- Optinose Investors Relation – https://ir.optinose.com/
- Fourth Quarter and Full Year 2023 Financial Results – https://ir.optinose.com/news-releases/news-release-details/optinose-reports-fourth-quarter-and-full-year-2023-financial
- XHANCE future development – https://www.optinose.com/research-and-development/xhance-future-development/
- Marketwatch analyst estimates – https://www.marketwatch.com/investing/stock/optn/analystestimates?mod=mw_quote_tab
- Yahoo finance analysis – https://finance.yahoo.com/quote/OPTN/analysis
Related Links:
Fundamental Analysis of Discover Financial Services (DFS)
Fundamental Analysis of Spirit AeroSystems (SPR)
Fundamental Analysis of Ocugen Inc (OCGN)
Fundamental Analysis of Mobileye (MBLY)