Geron Corporation (GERN), a late-stage biopharmaceutical company developing a first-in-class telomerase inhibitor called imetelstat for treating hematologic malignancies, recently released its business highlights and financial results for the fourth quarter and full year 2023. Here’s a breakdown of the key points:
2023 Achievements
- Positive top-line results from the Phase 3 IMerge trial evaluating imetelstat for treating transfusion-dependent lower-risk myelodysplastic syndromes (TD LR-MDS).
- U.S. Food and Drug Administration (FDA) acceptance of the New Drug Application (NDA) for imetelstat in TD LR-MDS, with a PDUFA target action date of June 16, 2024.
- European Medicines Agency (EMA) validation of the Marketing Authorization Application (MAA) for imetelstat in the same proposed indication as the NDA.
- Fifty percent enrollment achieved in the Phase 3 IMpactMF trial investigating imetelstat in relapsed/refractory myelofibrosis (R/R MF).
- Appointment of key personnel to strengthen the leadership team for a potential commercial launch.
Anticipated Milestones
- Potential U.S. commercial launch of imetelstat in the first half of 2024, subject to FDA approval.
- Review of the imetelstat MAA in the EU expected to be completed in early 2025.
- Interim analysis from the Phase 3 IMpactMF trial in R/R MF expected in the first half of 2025.
Commercial Readiness
- (GERN) has completed pre-commercial activities such as securing a brand name for imetelstat, establishing distribution networks, and building commercial and medical affairs teams.
- The company projects its current resources to be sufficient to fund operations into the third quarter of 2025, considering potential U.S. sales of imetelstat and additional funding sources.
Financials
- Geron ended 2023 with $378.1 million in cash and equivalents, bolstered by financing activities in 2023.
- The company projects these resources, along with anticipated revenue and loan access, to be sufficient for operations until Q3 2025.
- Revenues in 2023 were minimal, primarily from royalties on cell-based research products from divested assets.
- Operating expenses increased in 2023 due to higher clinical trial costs, commercial preparation activities, and personnel growth for a potential commercial launch.
Projected 2024 Financial Guidance
- Company expects total operating expenses in 2024 to be in the range of $270 million to $280 million.
- These expenses include costs for regulatory processes, ongoing clinical trials, commercial launch activities, and headcount growth.
- The Company plans to grow its workforce to approximately 270 employees by year-end 2024.
Strengths
- Promising Pipeline: Geron’s lead candidate, imetelstat, has shown positive results in clinical trials for treating transfusion-dependent lower-risk myelodysplastic syndromes (TD LR-MDS) and is under review by the FDA with a PDUFA date of June 16, 2024. Additionally, they are conducting Phase 3 trials for imetelstat in relapsed/refractory myelofibrosis (R/R MF).
- Experienced Management Team: It recently appointed new members to its board and executive team who bring extensive experience in commercializing drugs and navigating the regulatory process.
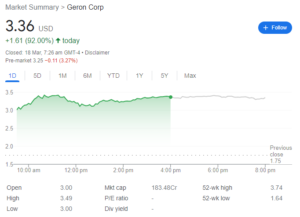
- Strong Financial Position: With over $378 million in cash and equivalents, it has sufficient resources to fund operations into Q3 2025, assuming a U.S. launch of imetelstat in mid-2024.
- Intellectual Property: Geron holds exclusive rights to imetelstat, giving them a potential first-mover advantage in this therapeutic area.
Weaknesses
- Limited Product Portfolio: Geron’s success is heavily dependent on the approval and commercialization of imetelstat. If imetelstat fails to gain regulatory approval or underperforms commercially, it could significantly impact the company’s future.
- Lack of Commercial Experience: It is a late-stage clinical biopharmaceutical company and has limited experience in commercializing drugs. They are currently building their commercial infrastructure in preparation for a potential U.S. launch of imetelstat.
- Competition: Geron faces competition from other pharmaceutical companies developing treatments for TD LR-MDS and R/R MF.
Opportunities
- Large Market Potential: The market for treatments for TD LR-MDS and R/R MF is significant and growing. If approved, imetelstat could capture a significant share of this market.
- Potential for Additional Indications: Imetelstat’s mechanism of action may be applicable to other hematologic malignancies beyond TD LR-MDS and R/R MF, opening doors for future development.
- Partnerships: Geron could potentially partner with other pharmaceutical companies to expand the reach and development of imetelstat.
Threats
- Regulatory Delays or Setbacks: The FDA or EMA approval process could be delayed or imetelstat could be rejected, impacting Geron’s timeline and finances.
- Pricing and Reimbursement Challenges: Geron will need to establish competitive pricing and navigate the complex reimbursement landscape to ensure market access for imetelstat.
- Clinical Trial Failures: The ongoing Phase 3 trials for imetelstat in R/R MF could fail to meet their endpoints, jeopardizing Geron’s future prospects.
Conclusion
Geron is at a pivotal point with a promising drug candidate and progress towards commercialization. However, their success hinges on navigating the challenges associated with late-stage clinical trials, regulatory hurdles, and navigating a competitive market. By leveraging their strengths and capitalizing on the available opportunities, Geron has the potential to become a major player in the treatment of hematologic malignancies.
Disclaimer: This blog post is for informational purposes only and should not be considered financial advice. It’s recommended to consult with a financial professional before making any investment decisions.
Additional Resources
- Geron Corporation Technicals on TradingView – https://in.tradingview.com/symbols/NASDAQ-GERN/technicals/
- Fourth Quarter and Full Year 2023 Financial Results – https://ir.geron.com/investors/press-releases/press-release-details/2024/Geron-Corporation-Reports-Business-Highlights-and-Fourth-Quarter-and-Full-Year-2023-Financial-Results/default.aspx
- February 2024 Corporate Presentation – https://s201.q4cdn.com/710325604/files/doc_presentations/2024/02/Geron-Corporate-Deck-February-2024-FINAL.pdf
Related Articles
Related Articles:
Fundamental Analysis of Discover Financial Services (DFS)
Fundamental Analysis of Laird Superfood (LSF)
Fundamental Analysis of Ocugen Inc (OCGN)
Fundamental Analysis of Mobileye (MBLY)